Northumbria expands results day support for students
Northumbria University is expanding and enhancing the support it provides to students receiving…
International
Northumbria’s global footprint touches every continent across the world, through our global partnerships across 17 institutions in 10 countries, to our 277,000 strong alumni community and 150 recruitment partners – we prepare our students for the challenges of tomorrow. Discover more about how to join Northumbria’s global family or our partnerships.
View our Global FootprintBusiness
The world is changing faster than ever before. The future is there to be won by organisations who find ways to turn today's possibilities into tomorrows competitive edge. In a connected world, collaboration can be the key to success.
More on our Business ServicesResearch
Northumbria is a research-rich, business-focused, professional university with a global reputation for academic quality. We conduct ground-breaking research that is responsive to the science & technology, health & well being, economic and social and arts & cultural needs for the communities
Discover more about our ResearchAlumni
Northumbria University is renowned for the calibre of its business-ready graduates. Our alumni network has over 253,000 graduates based in 178 countries worldwide in a range of sectors, our alumni are making a real impact on the world.
Our AlumniWhat’s in a food health claim?
Iain Brownlee 22/05/23
We currently live in an information-rich age where access to both primary data and informed or uninformed opinion is unprecedented. One of the common cultural memes is that what we are told to eat is constantly changing. This perceived issue would certainly make it difficult to make informed dietary choices.
A key doctrine of nutritional science is the need for careful evaluation of all available evidence to inform decision-making related to dietary intake and health. A great deal of information that we are exposed to related to food, diet and health is not evidence-based. Our own implicit biases are also likely to drive food choices we make on a daily basis. As common examples of seemingly confusing media tropes, you’re probably going to be more driven to believe that eggs are good or bad for health, or tea is “healthier” than coffee, not based on a careful evaluation of available evidence, but based on your own existing preferences. In each example above, a binary choice is made without a consideration that the truth could lie somewhere in between.
A complex, but still perhaps ultimately binary, approach to decision-making based on scientific evidence in the field of nutrition is an evaluation of whether claims can or cannot be made of food products or ingredients. Such evaluations are carried out by expert scientific committees around the world. In the UK, the laws relating to health claims for foods and supplements are currently based on existing European laws, originally informed by decisions of the European Food Safety Authority (EFSA). A UK-specific committee (the UK Nutrition and Health Claims Committee) to make national decisions is currently in development. Without changes to the food laws, UK expert committees would make their decisions in the same way the EFSA committees have in the past.
Sounds lovely. Why should I care?
The major aims of any initiative to define and enforce standards around claims on the impacts of food on health is to protect and inform consumers in their food choices. So while the approach to decision-making is definitely not of interest, being aware that foods can make claims based on their nutrient content (e.g. “high fibre” or “low saturated fat”) or the benefit that inclusion of a specific ingredient (e.g. beta-glucans and maintenance of normal blood glucose concentrations) may have on health probably is important in bypassing the need to process the complex and conflicting information that might be the basis of a statement being allowed or disallowed on a given product. As with other areas that impact on personal or public health, belief in the scientific process behind the final decision-making will not be absolute across everyone from industry, public and even research sectors. The approach of evaluation of new health claims is one based on soundness of the claim, in a pre-determined manner to limit the risk of bias and with consideration of the totality of available evidence (with a particular focus on studies in humans to make a claim on impacts on human health).
What else do I need to know?
EFSA have been pretty transparent about their process. They have made the outcomes of almost all submitted claims available online and searchable and have also published a series of summaries to support companies in targeting future claim submissions (through their journal). Many claims have not made it past the early stages of evaluation. If an ingredient or food that is not well-defined, then a claim is not assessable. Recent examples would include “organic foods” referring to wide range of products in every food group. As such, they cannot solely be considered one entity for the purpose of health claims evaluation (as, for example, an organic tomato, organic lamb and organic milk could all have very different impacts on a single health outcome). Similarly, if a claim not deemed to relate to a health effect, then no further evaluation is carried out. So a claim on changing the composition of the gut microbiota or skin appearance would be excluded as the effect is not directly on the human, whereas an impact on improving gut symptoms or benefitting skin barrier function could be considered as the basis for a health claim. Only if these first two hurdles are overcome would the evidence of an impact of consuming the food or ingredient on health be evaluated.
What should I watch out for?
As mentioned above, we are all programmed to work based on bias, so something that sounds like an easier or quicker way to improving health than changing all aspects of our diet and lifestyle is something most people would want to believe to be true. Approved nutrition claims and health claims read very different from marketing messaging, so spotting the difference should be relatively easy. Approved claims are eminently less dynamic but perhaps much more important to process and base your purchasing decisions on. The British Dietetic Association note that dietary regimes or supplements with evidence solely based on testimonials should be viewed very cautiously: A statement that “This worked for me” is very different to “Consuming X servings of product Y per day will lead to [specified improvement in health outcome Z]” – and possible less likely to motivate someone to choose . Generic statements that do not link a given product to health are not contravening any regulations related to food standards or advertising, even if they are strictly unevidenced. Something along the lines of “Have you ever wondered…?” or “Many people believe that…” is often not making a statement based on evidence. Less obvious examples might be making a fairly generic claim based on the composition of a product (e.g High in calcium. Calcium is important for digestive enzyme activity), then messaging around another feature of the product that links to the generic claim and allows it to be sold at a premium. This is where consumers need to read the fine print carefully, as they would if purchasing financial, or any other, products.

Northumbria University is expanding and enhancing the support it provides to students receiving…

With the global population expected to reach 10 billion by 2050 and crop yields declining annually,…

An Assistant Professor at Northumbria University won the Royal Society of Chemistry’s Award…
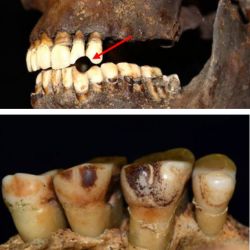
Researchers from Northumbria University have discovered that smokers have tell-tale signs of…

Northumbria University has been named Higher Education Institution of the Year at a prestigious…

Two biomedical sciences researchers from Northumbria University have been awarded grants to…

The Spring 2025 edition of Northumbria University’s newspaper is available to collect on campus…

Scientists have discovered that flies can demonstrate play-like behaviour – the first time…